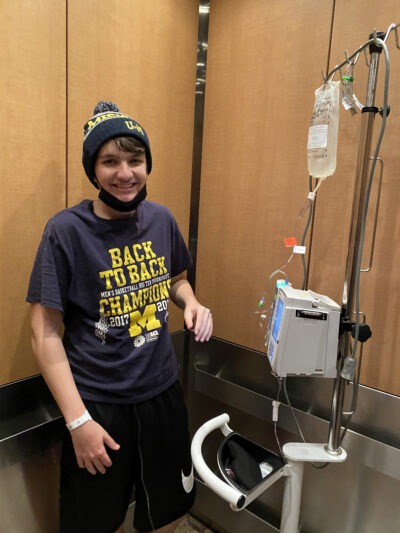
Atlanta Braves middle reliever Dylan Lee is the John Foy & Associates Strong Arm Pitcher of the Month for June 2025. Lee appeared in 11 games, tallied one save, and threw 12.2 strong innings without giving up an earned run. Lee struck out 15 hitters while walking one, and opposing runners failed to reach base …